abbreviated electron configuration|Noble Gas Configuration : iloilo 119 rows — Mar 23, 2023 — This web page provides a table of electron configuration chart for all elements, including shorthand and full forms. It also shows the electron shell arrangement for each element. Do you need to convert Central European Time (CET) to India Standard Time (IST) for your travel, business, or personal needs? Use this easy-to-use, modern time zone converter to quickly compare the time difference between CET and IST. You can also explore other time zones and locations with World Time Buddy, the ultimate tool for time travelers.
PH0 · Writing Condensed/Abbreviated Electron Configurations
PH1 · Noble Gas Configuration
PH2 · List of Electron Configurations of Elements
PH3 · Electron Configuration Chart of All Elements (Full Chart)
PH4 · Electron Configuration
PH5 · Chem – Abbreviated Electron Configurations
PH6 · Chem – Abbreviated Electron Configurat
PH7 · Abbreviated Electron Configurations
PH8 · 6.8: Electron Configurations
PH9 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH10 · 2.6: Electron Configurations
Tuition Fees: P 18,000-20,000 per semester. P 36,000-40,000 per year Last updated: November 2018 BS in Electrical Engineering . The data provided in this page was collected from University of Mindanao's website, other internet sources, as well as by calling or emailing the school's representatives.
abbreviated electron configuration*******119 rows — Mar 23, 2023 — This web page provides a table of electron configuration chart for all elements, including shorthand and full forms. It also shows the electron shell arrangement for each element.Electronic configuration [Rn] 5f 14 6d 10 7s 2 7p 6: Crystal structure (predicted) FCC .Learn how to write abbreviated electron configurations for atoms using a periodic table. Follow the steps to find the noble gas, the outer-electron configuration and the sublevels for each element.
Dis 20, 2020 — To write condensed electron configurations (also called abbreviated electron configurations) for elements we first write the full electron configuration for the element. Once .119 rows — Hun 14, 2015 — Learn how to write the electron configuration of each .
Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron .
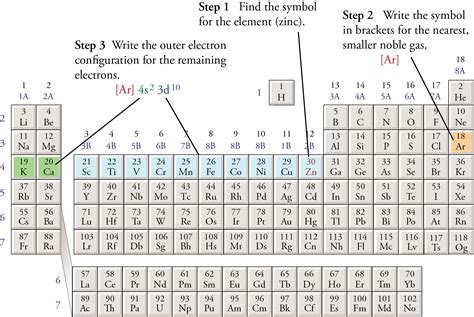
Mar 27, 2011 — Learn how to shorten complete electron configurations using the nearest noble gas above the element. See examples, video, and practice problems with orbital and periodic tables.Learn how to construct the electron configuration of an element by following the aufbau principle and Hund's rule. See examples of orbital diagrams and abbreviated electron configurations for .Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in a .Hul 27, 2021 — Learn how to write a noble gas configuration, a shorthand method of writing an atom’s electron configuration. See examples, steps, and a list of noble gas configurations for all 118 elements.What are electron configurations? Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the .The electron configurations for Cations are also made based on the number of electrons but there is a slight difference in the way they are configured. First you should write their normal electron configuration and then when you remove .In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule . The first excited state is obtained by promoting a 3s electron to the 3p subshell, to obtain the 1s 2 2s 2 2p 6 3p 1 configuration, abbreviated as the 3p level. Atoms can move from one configuration to another by .abbreviated electron configurationHul 20, 2022 — Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”). Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p, 6; d, 10; and f, 14 (Table \(\PageIndex{2}\)).Follow these steps to write abbreviated electron configurations. Step 1 Find the symbol for the element on a periodic table.. For example, to write an abbreviated electron configuration for zinc atoms, we first find Zn on the periodic table (see below).Step 2 Write the symbol in brackets for the noble gas located at the far right of the preceding horizontal row on the table.
A Noble Gas is a group of elements that in their standard state have a filled electron cloud.. These elements are found in the 18th column of the periodic table and include Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn). They are all odourless and colourless mono-atomic elements. Because these elements are already electron stable and do not need .Therefore, the abbreviated electron configuration of sodium is [Ne]3s 1 (the electron configuration of neon is 1s 2 2s 2 2p 6, which can be abbreviated to [He]2s 2 2p 6). Electron Configurations are useful for: Determining the valency of an element.Noble Gas Configuration Abr 28, 2016 — The abbreviated electron configurations uses Noble gas configurations, which have full electron shells, to describe the electronic structure of later elements. He (Z=2):1s^2 Ne (Z=10):1s^(2)2s^(2)2p^6 Ar(Z=18):1s^(2)2s^(2)2p^(6)3s^(2)3p^6 Now we know that on the basis of these fully occupied electronic configurations, the Noble Gases are supremely .Hun 27, 2024 — Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for .abbreviated electron configuration Noble Gas Configuration Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 .
Ene 30, 2015 — This lesson discusses how the Noble Gas element configurations can be used as a "shortcut" to create an abbreviated electron configuration; these are especia.
Mar 25, 2023 — The arrangement of electrons in mercury in specific rules in different orbits and orbitals is called the electron configuration of mercury. The electron configuration of mercury is 4f 14 5d 10 6s 2, if the electron .

Mar 25, 2023 — The arrangement of electrons in mercury in specific rules in different orbits and orbitals is called the electron configuration of mercury. The electron configuration of mercury is 4f 14 5d 10 6s 2, if the electron .From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number n and their value of l . The electron configurations of the elements are presented in Figure \(\PageIndex{2}\), which lists the orbitals in the order in which they are filled. .In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next .Abr 10, 2023 — Figure \(\PageIndex{5}\): A core-abbreviated electron configuration (right) replaces the core electrons with the noble gas symbol whose configuration matches the core electron configuration of the other element.
Hun 20, 2023 — The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the .Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written (here is an explanation why). Therefore we have 1s 2 2s 2 2p 6 3s 2 3p 6 3d .Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Mar 26, 2020 — What is the electron configuration and orbital diagram of: Na + P 3– Al 2 + Fe 2 + Sm 3 + Solution. First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also .
City of Sweetwater Building Department Address: 1701 NW 112th Ave, Miami, FL 33172 All City Permits is a full service permit expediter / runner with many years of experience expediting permits and code violation repair in South Florida, Miami Dade and Broward.. All City Permits exists to help ease the permit approval process and save time and money .
abbreviated electron configuration|Noble Gas Configuration